A 60-year-old woman with an asymptomatic left lacrimal gland mass found incidentally
Digital Journal of Ophthalmology 2017
Volume 23, Number 4
October 18, 2017
DOI: 10.5693/djo.03.2017.07.001
Volume 23, Number 4
October 18, 2017
DOI: 10.5693/djo.03.2017.07.001
Download PDF
Other past pertinent oncologic and ophthalmologic history included treatments for the maxillary sinus adenocarcinoma with multiple surgical resections, including a maxillectomy and high-dose radiation of 60 Gy to the nasal cavity and 50 Gy to the right and left neck lymph nodes, and repeated proton beam therapy to the sinonasal region for a local recurrence. The patient’s ocular history was significant for a bilateral dacryocystorhinostomy and left conjunctivodacryocystorhinostomy for nasolacrimal duct blockage and canalicular stenosis secondary to treatments for her sinonasal cancer. Additionally, she was diagnosed with iritis of the right eye 5 months prior to presentation by her local ophthalmologist that had resolved with topical steroids and was felt to be idiopathic in nature.
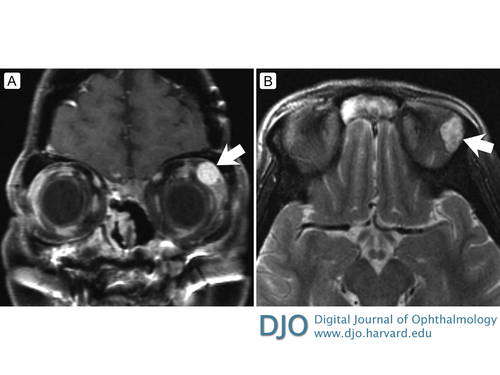
Figure 1
A, T1-weighted post-contrast coronal magnetic resonance imaging (MRI) demonstrating a well, circumscribed homogeneous mass in the left superolateral orbit. B, T2-weighted precontrast axial MRI demonstrating heterogeneous signal consistent for schwannoma.
A, T1-weighted post-contrast coronal magnetic resonance imaging (MRI) demonstrating a well, circumscribed homogeneous mass in the left superolateral orbit. B, T2-weighted precontrast axial MRI demonstrating heterogeneous signal consistent for schwannoma.
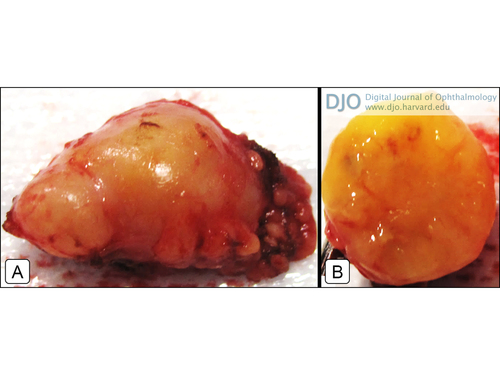
Figure 2
A, Gross surgical specimen showing the appearance of the lacrimal gland mass. B, Gross specimen in cross section.
A, Gross surgical specimen showing the appearance of the lacrimal gland mass. B, Gross specimen in cross section.
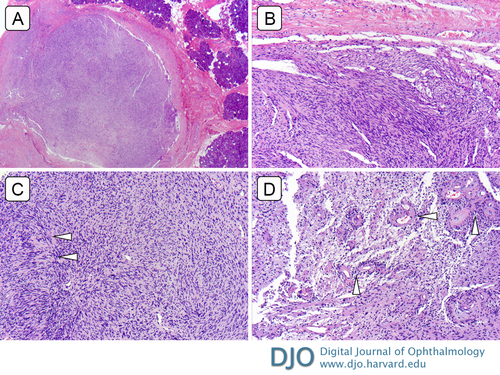
Figure 3
Lacrimal gland schwannoma. A, Scanning magnification reveals an encapsulated spindle cell tumor with areas of hyper- and hypo-cellularity adjacent to glandular tissue (hematoxylin-eosin [H&E] original magnification ×40). B, Spindle cell tumor comprised of areas of relative hypercellular Antoni A areas (H&E ×200). C, with Verocay body formation (arrowheads; H&E ×200). D, Antoni B area consisting of loose arrangement of spindle cells in a myxoid background with hyalinized thick-walled vessels (arrowheads; H&E ×200).
Lacrimal gland schwannoma. A, Scanning magnification reveals an encapsulated spindle cell tumor with areas of hyper- and hypo-cellularity adjacent to glandular tissue (hematoxylin-eosin [H&E] original magnification ×40). B, Spindle cell tumor comprised of areas of relative hypercellular Antoni A areas (H&E ×200). C, with Verocay body formation (arrowheads; H&E ×200). D, Antoni B area consisting of loose arrangement of spindle cells in a myxoid background with hyalinized thick-walled vessels (arrowheads; H&E ×200).
Schwannomas are benign, peripheral nerve sheath tumors, composed of neural crest–derived Schwann cells; they comprise 0.7%-2.3% of orbital tumors.(10,11) It commonly manifests along the supraorbital branch of the frontal nerve.(12) We postulate that our patient had an extension of her schwannoma from the lacrimal branch of the ophthalmic division of the trigeminal nerve.
Schwannomas classically consist of spindle cells arranged as hypercellular and alternating hypocellular regions known as Antoni A and Antoni B, respectively.(13) This biphasic phenomenon underlies the classic signal heterogeneity on T2-weighted imaging, as demonstrated in Figure 1B.(14,15) There can also be palisading arrangements of nuclei known as Verocay bodies.
The clinical and radiologic diagnosis of the lacrimal gland tumor in our patient was further hindered by her complex history of an aggressive primary adenocarcinoma of the maxillary sinus and local radiation. Our presumptive diagnosis was a benign epithelial tumor or metastatic disease. The gross specimen after surgical resection had a yellow appearance with a potatolike consistency characteristic of a pleomorphic adenoma. However, it was not until histopathologic assessment that a diagnosis of Schwannoma of the lacrimal gland was made. In addition to nonspecific S100 protein and glial fibrillary acid protein expression in schwannomas, markers such as calretinin, podoplanin, and SOX10 may also be useful in elucidating the diagnosis.(16-18) In a recent review of the literature, Yamanaka and Hayano reported 28 cases of radiation-induced schwannomas.(19) Because the primary tumor was on the left maxillary sinus and nasal cavity, the radiation field may have included the left orbit. Since the schwannoma was not present on imaging prior to radiation, it is possible that adjacent surrounding structures may have been affected thereby promoting tumorogenesis of this rare, neoplastic variety.
Our case demonstrates that, although rare, schwannoma of the lacrimal gland should be considered on the differential diagnosis of a well-circumscribed lacrimal gland mass. Gross total surgical resection is the appropriate treatment particularly if there are signs of growth or concerns for other more malignant diseases, as was the case in our patient with a history of significant malignant carcinoma of the maxillary sinus.
2. von Holstein SL, Therkildsen MH, Prause JU, Stenman G, Siersma VD, Heegaard S. Lacrimal gland lesions in Denmark between 1974 and 2007. Acta Ophthalmologica 2013;91:349-54.
3. Esmaeli B. Ophthalmic Oncology. New York: Springer; 2011.
4. Bajaj MS, Mehta M, Sen S, et al. Schwannoma: an unusual lacrimal gland tumor in a child. J Pediatr Ophthalmol Strabismus 2010;47:380-1.
5. De silva DJ, Tay E, Rose GE. Schwannomas of the lacrimal gland fossa. Orbit 2009;28:433-5.
6. Kashyap S, Pushker N, Meel R, et al. Orbital schwannoma with cystic degeneration. Clin Experiment Ophthalmol 2009;37:293-8.
7. Nadkarni T, Goel A. A trigeminal neurinoma involving the lacrimal nerve: case report. Br J Neurosurg 1999;13(1):75-6.
8. Rose GE, Wright JE. Isolated peripheral nerve sheath tumours of the orbit. Eye (Lond) 1991;5(Pt 6):668-73.
9. Singh U, Sukhija J, Raj S, Radotra BD, Gupta A. Calcification in schwannoma of the lacrimal gland region. Eye (Lond) 2004;18:218.
10. Karcioglu ZA. Orbital Tumors: Diagnosis and Treatment. New York, Springer; 2004.
11. Sweeney AR, Gupta D, Keene CD, et al. Orbital peripheral nerve sheath tumors. Surv Ophthalmol 2017;62:43-57.
12. Rootman J. Diseases of the Orbit: A Multidisciplinary Approach. Philadelphia: Lippincott Williams & Wilkins; 2003.
13. Wippold FJ 2nd, Lubner M, Perrin RJ, Lämmle M, Perry A. Neuropathology for the neuroradiologist: Antoni A and Antoni B tissue patterns. AJNR Am J Neuroradiol 2007;28(9):1633–1638
14. Skolnik AD, Loevner LA, Sampathu DM, et al. Cranial nerve schwannomas: diagnostic imaging approach. Radiographics 2016;36:150199.
15. Shen WC, Yang DY, Ho WL, Ho YJ, Lee SK. Neurilemmoma of the oculomotor nerve presenting as an orbital mass: MR findings. AJNR Am J Neuroradiol 1993;14:1253-4.
16. Fine SW, McClain SA, Li M. Immunohistochemical staining for calretinin is useful for differentiating schwannomas from neurofibromas. Am J Clin Pathol 2004;122:552-9.
17. Jokinen CH, Dadras SS, Goldblum JR, van de Rijn M, West RB, Rubin BP. Diagnostic implications of podoplanin expression in peripheral nerve sheath neoplasms. Am J Clin Pathol 2008;129:886–893.
18. Nonaka D, Chiriboga L, Rubin BP. Sox10: a pan-schwannian and melanocytic marker. Am J Surg Pathol 2008;32:1291-8.
19. Yamanaka R, Hayano A. Radiation-induced schwannomas and neurofibromas: a systematic review. World Neurosurg 2017;104:713-22.