A 24-year-old woman with rapidly progressing vision loss
Digital Journal of Ophthalmology 2017
Volume 23, Number 1
January 15, 2017
DOI: 10.5693/djo.03.2016.10.001
Volume 23, Number 1
January 15, 2017
DOI: 10.5693/djo.03.2016.10.001
Download PDF
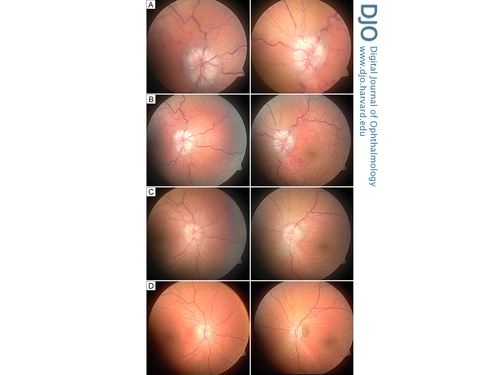
Figure 1
Fundus images of the right eye (left column) and left eye (right column) at acute presentation (A), and at 1 week (B), 6 weeks (C), and 18 months (D) after surgery.
Fundus images of the right eye (left column) and left eye (right column) at acute presentation (A), and at 1 week (B), 6 weeks (C), and 18 months (D) after surgery.
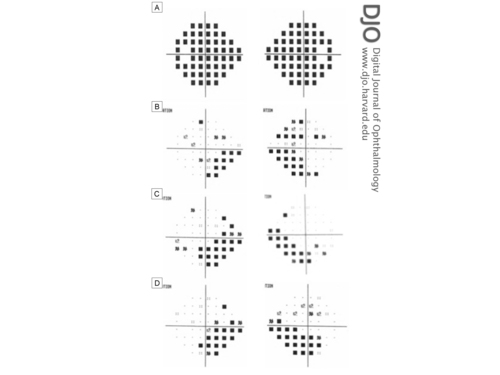
Figure 2
Humphrey’s 24-2 visual field total and/or pattern deviation results of the right eye (right column) and left eye (left column). A, Total deviation at acute presentation (pattern deviations not shown due to severely depressed fields). B, Pattern deviation at 6 weeks after surgery. C, Pattern deviation at 4 months after surgery. D, Pattern deviation at 1 year after surgery
Humphrey’s 24-2 visual field total and/or pattern deviation results of the right eye (right column) and left eye (left column). A, Total deviation at acute presentation (pattern deviations not shown due to severely depressed fields). B, Pattern deviation at 6 weeks after surgery. C, Pattern deviation at 4 months after surgery. D, Pattern deviation at 1 year after surgery
Given the rapidly progressive visual loss despite maximal tolerated medical treatment, a lumboperitoneal shunt surgery was urgently performed. Opening pressure at the time of the procedure was not recorded. All medications were subsequently discontinued.
One week after surgery, the patient’s vision, diplopia, and headaches had improved. On examination, she had a best-corrected visual acuity of 20/25 in the right eye and 20/30 in the left eye. Pupils were equal and reactive. Ocular motility examination showed resolution of her abducens nerve palsy. Funduscopic examination showed improvement of the papilledema (Figure 1B), and visual field assessment exhibited improved visual fields bilaterally, with a mean deviation of −24.4 dB in the right eye and −18.5 dB in the left eye. Postoperative follow-up at 1, 5, 9, 13, and 18 months demonstrated continued resolution of papilledema and retinal venular dilation and tortuosity (Figure 1C) with improvement of visual fields, with a mean deviation of −13.8 dB in the right eye and −8.4 dB in the left eye (Figure 2D). At final follow-up, 18 months postoperatively, visual acuity had stabilized at 20/25 in each eye. There was mild pallor of both optic nerves, suggesting permanent optic nerve damage. Visual field testing showed persistent inferonasal deficits bilaterally. On follow-up examination, there was mild peripapillary hyperpigmentation nasal to the left optic nerve but no evidence of choroidal neovascularization.
The overall incidence of IIH is 1-3 per 100,000, but it rises dramatically to 20 per 100,000 among female patients who are obese or have experienced recent weight change.(1,2) Fulminant IIH is a particularly acute and rapidly progressive form of IIH, with an incidence of 2.2% to 2.9% of all new IIH cases.(3)
Normally, vision loss in IIH is secondary to chronic papilledema;(4) however, progressive vision loss is rare and may indicate secondary causes of increased ICP, such as a meningeal process or venous sinus thrombosis.(5,6) Fulminant IIH is an acutely severe and rapidly progressive form of IIH, with resultant permanent visual sequelae.(3,7-13) It is defined as acute onset of signs and symptoms of intracranial hypertension; (<4 weeks between onset of initial symptoms and severe visual loss), rapid worsening of vision loss over several days, and a normal MRI and MRV (or CT venogram).(3) There is limited literature on fulminant IIH.(8-12)
The pathophysiology of IIH remains unknown. Vision loss in papilledema is thought to be secondary to elevated CSF pressure transmitted to the anterior optic nerve sheath, resulting in axoplasmic flow stasis and subsequent intraneuronal ischemia.(14) It is unclear why the condition manifests more rapidly and severely in some patients. The clinical characteristics of fulminant IIH are similar to those in the nonacute form.(3)
The treatment of fulminant IIH aims to alleviate symptoms and to preserve visual function. Fulminant IIH requires surgical intervention. Close observation and temporizing measures, such as repeat lumbar punctures, lumbar drain placement, acetazolamide and intravenous steroids are indicated prior to surgery.(3) The patient in our case was treated with 1 g daily acetazolamide, because she was unable to tolerate a higher dose. Although patients can be treated with up to 4 g daily of acetazolamide, few patients can actually tolerate the side effect profile.
The use of corticosteroid therapy in the context of IIH has been suggested to decrease CSF formation at high ICP, to increase CSF absorption, and to mitigate papilledema independent of any change in ICP.(15) Nevertheless, long-term use of steroids is not recommended due to well-established side effects.
Surgical management consists of CSF diversion procedures, namely, lumboperitoneal shunts and ventriculoperitoneal shunts, as well as optic nerve sheath fenestration (ONSF). In the United States, from 1988 to 2002, the frequency of CSF shunting procedures (lumboperitoneal and ventriculoperitoneal) for the treatment of IIH increased by 350%.(16) Recently, venous sinus stenting has been performed on selected patients with IIH, although this remains an area of debate.(17) To our knowledge, there have been no randomized, prospective trials undertaken to date that compare the efficacy and visual outcomes of these procedures in treating IIH.(18) In addressing the underlying etiology of increased ICP, CSF diversion techniques improve papilledema and reduce further visual loss; however, they do not restore visual loss.
ONSF has also been shown to prevent visual deterioration and even improve visual function in some patients with IIH.(19) However, up to one-third of patients with initially improved symptoms following ONSF experience worsening of visual acuity and visual fields over time. Thus, these patients require long-term follow-up, as deterioration of visual function may necessitate repeat procedures for ONSF failures. Overall, the decision to perform CSF diversion procedures or ONSF often depends on local availability and expertise as well as the predominance of the presenting symptoms. CSF shunting is often undertaken when headache is the primary presentation, whereas ONSF is carried out when vision loss is predominant.(20,21)
Regardless of the choice of procedure, there is consensus for rapid and definitive treatment in rapidly progressive and refractory cases of IIH, as delay in surgical intervention can lead to worse visual outcomes.(3,21) Thambisetty et al conducted the largest reported case series of fulminant IIH in 16 patients, who underwent surgical CSF shunting procedure or ONSF.(3) Visual function improved in 14 of 16 patients postoperatively, although 8 patients (50%) remained legally blind at last follow-up; visual fields remained severely altered in all cases. Notably, the 8 patients who remained legally blind had a median delay in surgical intervention of 6.5 days from the time of diagnosis (range, 3-37 days), whereas those that regained significant visual function had a median delay of only 2 days (range, several hours to 4 days), highlighting the importance of prompt and aggressive management.
Altogether, the acute progression and long-term visual sequelae of fulminant IIH in otherwise young healthy patients merits appropriate recognition, emergent neuro-ophthalmic evaluation, and possible surgical intervention. Surgical management is recommended in medically refractory cases with established progressive visual loss.
2. Durcan FJ, Corbett JJ, Wall M. The incidence of pseudotumor cerebri. Population studies in Iowa and Louisiana. Arch Neurol 1988;45:757-7.
3. Thambisetty M, Lavin PJ, Newman NJ, Biousse V. Fulminant idiopathic intracranial hypertension. Neurology 2007;68;229-32.
4. Friedman DI, Liu Gt, Digre KB: Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology 2013;81:1159-65.
5. Rush JA. Pseudotumor cerebri. Clinical profile and visual outcome in 63 patients. Mayo Clin Proc 1980;55:541-6.
6. Corbett JJ, Savino PJ, Thompson HS, et al. Visual loss in pseudotumor cerebri. Follow-up of 57 patients from five to 41 years and a profile of 14 patients with permanent severe visual loss. Arch Neurol 1982;39:461-74.
7. Heckmann JG, Stefan H, Groh MJ, Winterholler M, Neundörfer B. A rare cause of acute vision loss: pseudotumor cerebri: case report of course with recurrence after decompression of the optic nerve [in German]. Nervenarzt 1998;69:702-6.
8. Kidron D, Pomeranz S. Malignant pseudotumor cerebri: report of two cases. J Neurosurg 1989;71:443-5.
9. Groh MJ, Jünemann A. Papilledema and acute loss of vision in a patient with pseudotumor cerebri [in German]. Klin Monat Augenheilkunde 1999;215:315-8.
10. Pollock SC. Acute papilledema and visual loss in a patient with pseudo- tumor cerebri. Arch Ophthalmol 1987;105:752-3.
11. Malomo AO, Idowu OE, Shokunbi MT, Nwaorgu OG, Oluleye TS. Non-operative management of benign intracranial hypertension presenting with complete visual loss and deafness. Pediatr Neurosurg 2006;42:62-4.
12. Liu GT, Volpe NJ, Schatz NJ, Galetta SL, Farrar JT, Raps EC. Severe sudden visual loss caused by pseudotumor cerebri and lumboperitoneal shunt failure. Am J Ophthalmol 1996;122:129-31.
13. Liu GT, Glaser JS, Schatz NJ. High-dose methylprednisolone and acetazolamide for visual loss in pseudotumor cerebri. Am J Ophthalmol 1994;118:88-96.
14. Lee AG, Wall M. Papilledema: Are we any nearer to a consensus on pathogenesis and treatment? Curr Neurol Neurosci Rep 2012;12:334-9.
15. Digre KB, Corbett JJ. Idiopathic intracranial hypertension (pseudotumor cerebri): a reappraisal. Neurologist 2001;7:2-67.
16. Curry WT Jr, Butler WE, Barker FG II. Rapidly rising incidence of cerebrospinal fluid shunting procedures for idiopathic intracranial hypertension in the United States, 1988-2002. Neurosurgery 2005;57:97-108.
17. Ahmed R, Friedman DI, Halmagyi GM. Stenting of the transverse sinuses in idiopathic intracranial hypertension. J Neuro-Ophthalmol 2011;31:374-80
18. Lueck C, McIlwaine G. Interventions for idiopathic intracranial hypertension. Cochrane Database Syst Rev 2005(3):CD003434.
19. Brazis PW. Clinical review: the surgical treatment of idiopathic pseudotumour cerebri (idiopathic intracranial hypertension). Cephalalgia 2008;28:1361-73.
20. Spitze A, Malik A, Al-Zabudi N, Golnik K, Lee AG: Optic nerve sheath fenestration vs cerebrospinal diversion procedures: what is the preferred surgical procedure for the treatment of idiopathiv intracranial hypertension failing maximum medical therapy? J Neuro-Ophthalmol 2013;33:183-8.
21. Uretsky S. Surgical interventions for idiopathic intracranial hypertension. Curr Opin Ophthalmol 2009;20:451-5.