A 73-year-old man with congestion and mild proptosis of the left eye
Digital Journal of Ophthalmology 2016
Volume 22, Number 2
April 25, 2016
DOI: 10.5693/djo.03.2014.05.001
Volume 22, Number 2
April 25, 2016
DOI: 10.5693/djo.03.2014.05.001
Download PDF
The patient denied vision loss, double vision, headache, or other systemic symptoms. He had undergone bilateral orchidectomy and radiotherapy for prostate carcinoma 3 years earlier and was now on hormone therapy with bicalutamide. He had undergone thyroidectomy for hyperthyroidism 15 years previously and was now on levothyroxine. Ocular history was remarkable for uneventful cataract extraction in both eyes with IOL placement 20 years previously.
General examination did not reveal any abnormal cervical lymph nodes. Cranial nerve evaluation was within normal limits.
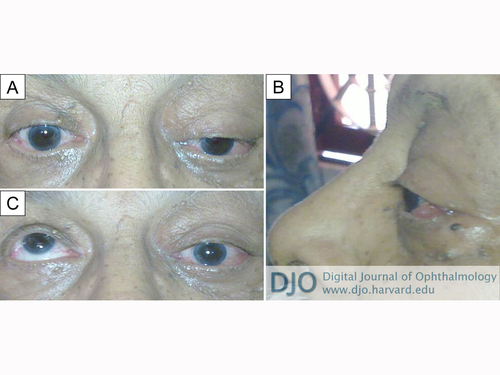
Figure 1
Clinical photograph of the patient at presentation. A, Left periorbital fullness and partial ptosis, with inferior displacement of the globe. B, Proptosis of left eye with temporal conjunctival congestion and chemosis. C, Complete loss of elevation in left eye.
Clinical photograph of the patient at presentation. A, Left periorbital fullness and partial ptosis, with inferior displacement of the globe. B, Proptosis of left eye with temporal conjunctival congestion and chemosis. C, Complete loss of elevation in left eye.
Fine-needle aspiration cytology of the mass was undertaken and a diagnosis of poorly differentiated carcinoma, possibly adenocarcinoma was made (Figure 3). PSA staining confirmed prostatic origin.
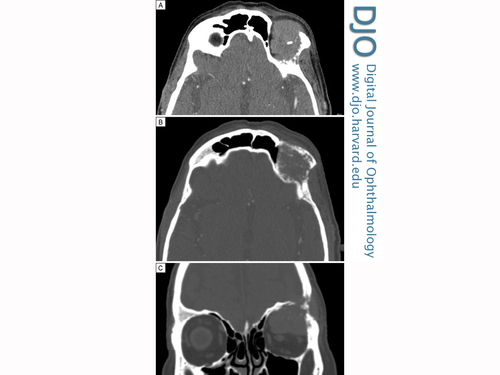
Figure 2
Computed tomography of the brain and orbits. Axial images (A, soft tissue window; B, bone window) showing enhancing lytic lesion (22 × 35 × 34 mm), with significant soft tissue component and calcification. Coronal reconstruction (C) showing the lesion eroding left frontal bone on the lateral wall of the orbit.
Computed tomography of the brain and orbits. Axial images (A, soft tissue window; B, bone window) showing enhancing lytic lesion (22 × 35 × 34 mm), with significant soft tissue component and calcification. Coronal reconstruction (C) showing the lesion eroding left frontal bone on the lateral wall of the orbit.
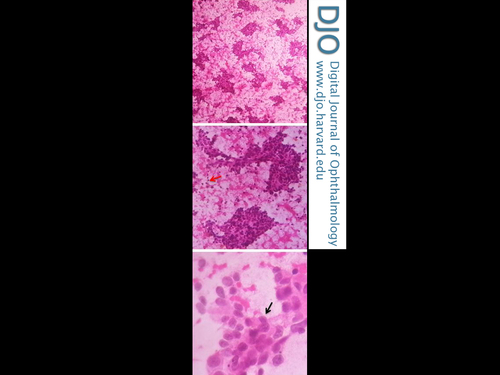
Figure 3
Fine-needle aspiration cytology showing round to polygonal cells having hyperchromatic pleomorphic nuclei, with indistinct nucleoli and moderate amount of eosinophilic cytoplasm (black arrow); scattered bare nuclei (red arrow) were also noted (H & E, original magnification ×40, ×100, ×400).
Fine-needle aspiration cytology showing round to polygonal cells having hyperchromatic pleomorphic nuclei, with indistinct nucleoli and moderate amount of eosinophilic cytoplasm (black arrow); scattered bare nuclei (red arrow) were also noted (H & E, original magnification ×40, ×100, ×400).
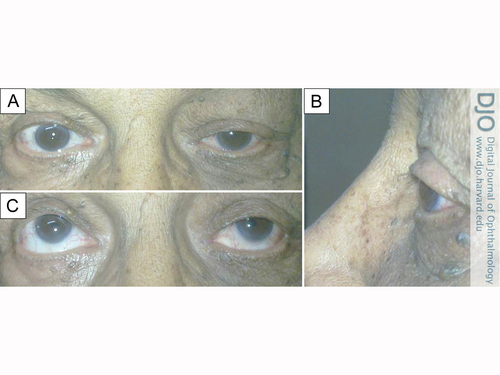
Figure 4
Clinical photographs 2 weeks after radiotherapy. A, Reduced lid edema and partial ptosis of left eye. B, Reduced proptosis and resolved conjunctival chemotic congestion of left eye. C, Moderate restriction of elevation in left eye.
Clinical photographs 2 weeks after radiotherapy. A, Reduced lid edema and partial ptosis of left eye. B, Reduced proptosis and resolved conjunctival chemotic congestion of left eye. C, Moderate restriction of elevation in left eye.
Another differentiating feature is that prostate metastases usually present as osteoblastic lesions in contrast to other orbital metastases which present as osteolytic lesions.(4,5) However, in the terminal stages of the disease, osteolytic and mixed osteoblatic-osteolytic lesions are seen.(6) A characteristic feature suggestive of prostatic origin is a hyperostotic and spiculated lesion on CT scan.(7) Soft tissue involvement is rare and the PSA level is elevated in 99% cases of metastatic disease.(8,9) In contrast to the aforementioned typical features, the present case was unusual because the patient presented with osteolytic metastasis to the orbit with significant soft tissue involvement and low PSA values, necessitating a biopsy to confirm the diagnosis.
Tumor metastasis to the orbit from prostate cancer may occur through the general hematogenous route of the carotid/ophthalmic artery or through Batsons venous plexus, which transports tumor emboli from the prostate to the cerebral venous sinuses/ophthalmic vein.(10-12) Patients usually present with one or more clinical features, such as decreased visual acuity, ocular pain, proptosis, retinal detachment, presence of a mass, secondary glaucoma, and osteoblastic lesions of the orbital wall.(12) The treatment of prostatic metastases to the orbit is palliative (androgen ablation or local radiotherapy) and does not alter survival.(5)
Prostate cancer is the second most common cancer in men and the fifth most common cause of death from cancer in men worldwide.(13) Hence, clinicians should maintain a high index of suspicion of metastasis from prostate cancer in any elderly male who presents with even a mild conjunctival chemosis. This is especially relevant in patients with comorbid conditions that can lead to a similar clinical presentation. The present case highlights the fact that neither the osteolytic nature of the orbital lesion nor normal PSA levels can conclusively rule out orbital lesions secondary to prostate carcinoma and that early and accurate diagnosis may only be possible with a biopsy.
Literature Review
Pubmed, MEDLINE, Google Scholar, were all searched, without language restriction, on February 3, 2016, using the following terms: prostate carcinoma, orbital metastasis in prostate carcinoma, proptosis in orbital metastasis, PSA levels, vertebral veins AND prostate carcinoma.
2. Sher JH, Weinstock SJ. Orbital metastasis of prostatic carcinoma. Can J Ophthalmol 1983;18:248-50.
3. Wolter JR, Hendrix RC. Osteoblastic prostate carcinoma to the orbit. Am J Ophthalmol 1981;91:648-51.
4. Boldt HC, Nerad JA. Orbital metastases from prostate carcinoma. Arch Ophthal 1988;106:1403-8.
5. Patel AR, Olson KB, Pienta KJ. Proptosis and decreased vision secondary to prostate cancer orbital wall metastasis. Anticancer Res 2005;25: 3521-2.
6. Plesnicar S. The course of metastatic disease originating from carcinoma of the prostate. Clin Exp Metastasis 1985;3:103-10.
7. Vilenski L, Potti A, Mehdi SA. Unusual tumors involving the head and neck region: case 3, proptosis due to metastatic prostate cancer. J Clin Oncol 2001;19:4177-9.
8. Nayyar R, Singh P, Panda S, Kashyap S, Gupta N P. Proptosis due to "isolated" soft tissue orbital metastasis of prostate carcinoma. Indian J Cancer 2010;47:74-6
9. Lee DK, Park JH, Kim JH, et al. Progression of prostate cancer despite an extremely low serum level of prostate-specific antigen. Korean J Urol 2010;51:358-61.
10. Gandhi HA, Shah MK. An unusual case of orbital metastasis from adenocarcinoma of prostate. Hospital Physician 2005;41:41-3.
11. Baltogiannis D, Kalogeropoulos C, Loachim E, Agnantis N, Psilas K, Giannakopoulos X. Orbital metastasis from prostate carcinoma. Urol Int 2003;70:219-22.
12. Batson OV. The function of vertebral veins and their role in the spread of metastasis. 1940. Clin Orthop Relat Res 1995;312:4-9.
13. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available at http://globocan.iarc.fr, accessed February 2016.