A 61-year-old man with cystoid macular edema and chorioretinal folds after cataract surgery
Digital Journal of Ophthalmology 2017
Volume 23, Number 3
August 29, 2017
DOI: 10.5693/djo.03.2017.02.002
Volume 23, Number 3
August 29, 2017
DOI: 10.5693/djo.03.2017.02.002
Download PDF
His preoperative refraction was +7.00 +2.50 ×180 correcting to 20/30 in the right eye and +7.75 +1.50 ×029 correcting to 20/30 in the left eye. A 36 D AcrySof SN60WF (Alcon, Fort Worth, TX) intraocular lens (IOL) was implanted in each eye.
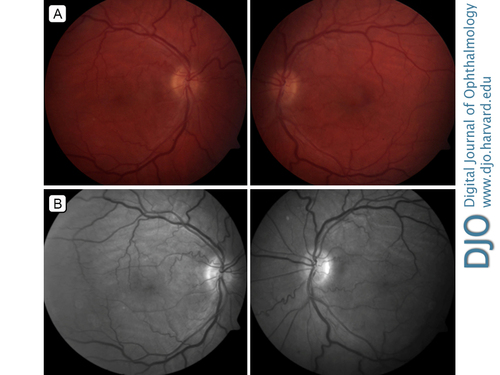
Figure 1
Fundus photographs (A) and red-free photographs (B) showing chorioretinal folds, venular dilation, and vessel tortuosity.
Fundus photographs (A) and red-free photographs (B) showing chorioretinal folds, venular dilation, and vessel tortuosity.
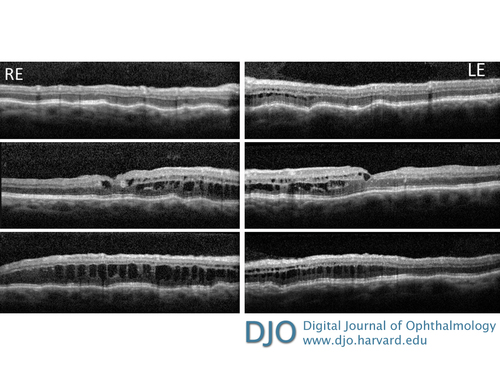
Figure 2
Optical coherence tomography showing choroidal folds and diffuse intraretinal cystoid changes in both eyes, with a central macular thickness of 441 µm in the right eye and 387 µm in the left eye.
Optical coherence tomography showing choroidal folds and diffuse intraretinal cystoid changes in both eyes, with a central macular thickness of 441 µm in the right eye and 387 µm in the left eye.
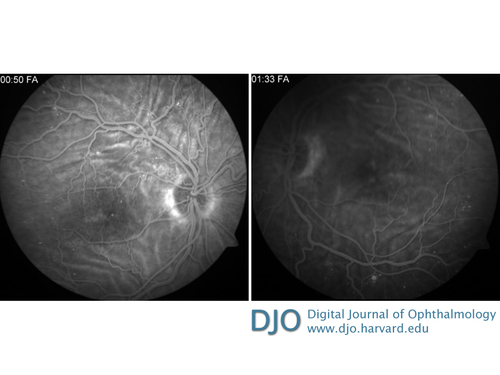
Figure 3
Fluorescein angiography showing alternating hypo- and hyperfluorescent lines in both eyes, corresponding to chorioretinal folds. There was mild early hyperfluorescence in the macula that increased during the course of the study, corresponding to macular edema. Pinpoint hyperfluorescent spots correspond to microaneurysms.
Fluorescein angiography showing alternating hypo- and hyperfluorescent lines in both eyes, corresponding to chorioretinal folds. There was mild early hyperfluorescence in the macula that increased during the course of the study, corresponding to macular edema. Pinpoint hyperfluorescent spots correspond to microaneurysms.
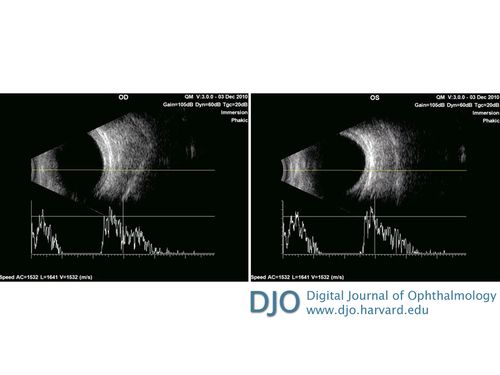
Figure 4
Ultrasound imaging showing retinal-choroidal-scleral thickness of 2.09 mm in the right eye and 2.36 mm in the left eye. A-scan overlay showing the short axial lengths of each eye, the right eye measuring 19.53 mm and the left eye measuring 19.48 mm.
Ultrasound imaging showing retinal-choroidal-scleral thickness of 2.09 mm in the right eye and 2.36 mm in the left eye. A-scan overlay showing the short axial lengths of each eye, the right eye measuring 19.53 mm and the left eye measuring 19.48 mm.
Nanophthalmos is a rare condition; a preliminary diagnosis can be made with a measurement of axial length <21 mm.(6) Presentation is normally bilateral, and patients can have slight enophthalmos and narrow palpebral fissures.(7) Other characteristics include severe hyperopia, increased retinal-choroidal-scleral thickness, a crowded anterior chamber, and chorioretinal folds.(5,6,8,9) The crowded anterior chamber of nanophthalmos is of particular concern, because it predisposes the patient to acute angle-closure glaucoma.(10) Additional complications arise from a thick scleral collagen, which can result in choroidal effusion, retinal detachment, and cystoid macular edema.(11)
Most cases of nanophthalmos are spontaneous, although familial types exist. Cases of autosomal recessive (AR) and dominant (AD) nanophthalmos have been attributed to mutations on chromosome 11.(7) AR nanophthalmos has been reported to involve the MFRP (membrane frizzled-related protein) gene. The MFRP protein is expressed in the retinal pigment epithelium and ciliary body and is important to later stages of ocular development.(6,7) It is thought that mutations in MFRP result in decreased ocular growth, leading to crowding and thickening of the retina and choroid, abnormal foveal development, and chorioretinal folds.(6,7,8,12) A gene TMEM98 (transmembrane protein 98) on chromosome 17 has also recently been implicated in a form of AD nanophthalmos.(13,14) Nanophthalmos can additionally be present in less common conditions such as oculo-dento-digital and Kenney Caffey syndromes.(7)
Nanophthalmos patients are at increased risk for cataract surgery complications. (2,9,15). Two recent studies have reported the complication rate of cataract surgery in nanophthalmos patients: Steijns et al reported a 27.9% complication rate (n = 43), whereas Day et al reported 15.5% complication rate (n = 103).(2,15) Surgical complications in nanophthalmos include choroidal effusion, zonular dehiscence, uveitis, malignant glaucoma, and cystoid macular edema.(2,9,15,16) It has been suggested that retinal-choroidal-scleral thickness be measured preoperatively in hyperopic eyes at risk of glaucoma to diagnose properly nanophthalmos and to allow more careful planning of surgery.(9) Preventative measures to be considered prior to cataract surgery include laser iridotomy, iridoplasty, trabeculectomy, and creation of scleral windows.(16) The use of intravenous mannitol to maintain pressure during surgery might also be considered.
2. Steijns D, Bijlsma W, Van der Lelij A. Cataract surgery in patients with nanophthalmos. Ophthalmology 2013;120:266-70.
3. MacKay C, Shek M, Carr R, Yanuzzi LA, Gouras P. Retinal degeneration with nanophthalmos, cystic macular degeneration, and angle closure glaucoma. a new recessive syndrome. Arch Ophthalmol 1987;105:366-71.
4. Olsen T, Palejwala N, Lee L, Bergstrom C, Yeh S. Chorioretinal folds: associated disorders and a related maculopathy. Am J Ophthalmol 2014;157:1038-47.
5. Yalçindag N, Atilla H, Bat?o?lu F. Optical coherence tomography findings of retinal folds in nanophthalmos. Case Rep Ophthalmol Med 2011; 2011:491894.
6. Sundin O, Dharmaraj S, Bhutto I, et al. Developmental basis of nanophthalmos: MFRP is required for both prenatal ocular growth and postnatal emmetropization. Ophthalmic Genetics 2008;29:1-9.
7. Traboulsi E. Nanophthalmos. In: Silva E, Sundin O, eds. Genetic Diseases of the Eye. Oxford University Press, 2012; chap 4.
8. Serrano JC, Hodgkins PR, Taylor DS, Gole GA, Kriss A. The nanophthalmic macula. Br J Ophthalmol 1998;82:276-9.
9. Wu W, Dawson D, Sugar A, et al. Cataract surgery in patients with nanophthalmos: results and complications. J Cataract Refract Surg 2004;30:584-90.
10. Kimbrough RL, Trempe CS, Brockhurst RJ, Simmons RJ. Angle-closure glaucoma in nanophthalmos. Am J Ophthalmol 1979;88:572-9.
11. Rao A, Padhi T, Jena A, Mandal S, Das T. Atypical features of nanophthalmic macula—a spectral domain OCT study. BMC Ophthalmol 2012;12:12.
12. Walsh M, Goldberg M. Abnormal avascular zone in nanophthalmos. Am J Ophthalmol 2007;143:1067-8.
13. Khorram D, Choi M, Roos B, et al. Novel TMEM98 mutations in pedigrees with autosomal dominant nanophthalmos. Mol Vis 2015;21:1017-23.
14. Awadalla MS, Burdon KP, Souzeau E, et al. Mutation in TMEM98 in a large white kindred with autosomal dominant nanophthalmos linked to 17p12-q12. JAMA Ophthalmol 2014;132:970.
15. Day A, MacLaren R, Bunce C, Stevens J, Foster PJ. Outcomes of phacoemulsification and intraocular lens implantation in microphthalmos and nanophthalmos. J Cataract Refract Surg 2013;39:87-96.
16. Sharon S, Grigg J, Higgins R. Nanophthalmos: ultrasound biomicroscopy and Pentacam assessment of angle structures before and after cataract surgery. J Cataract Refract Surg 2006;32:1052-5.