A 31-year-old man with bilateral blurry vision and floaters
Digital Journal of Ophthalmology 2015
Volume 21, Number 2
May 7, 2015
DOI: 10.5693/djo.03.2014.08.003
Volume 21, Number 2
May 7, 2015
DOI: 10.5693/djo.03.2014.08.003
Download PDF
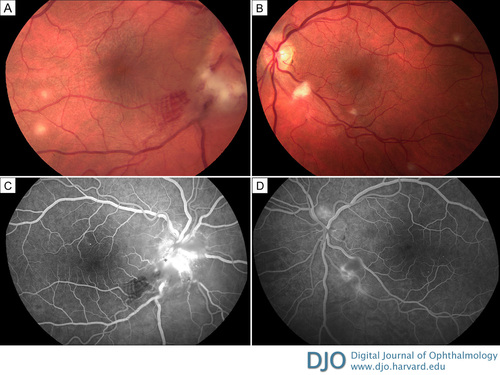
Figure 1
Fundus photographs of a 31-year-old man initially presenting with bilateral disc edema, in evidence more on the right (A) than the left (B), and multifocal deep retinal and choroidal yellowish infiltrates 100–300 μm in diameter. Representative fluorescein angiography of the right eye (at 40 seconds) shows prominent optic disc capillary dilation with hyperfluorescence and blockage at the site of the retinitis in the inferotemporal macula (C); the left eye (at 55 seconds) shows prominent optic disc capillary dilation as well as hypofluorescence at the site of the retinitis inferiorly (D).
Fundus photographs of a 31-year-old man initially presenting with bilateral disc edema, in evidence more on the right (A) than the left (B), and multifocal deep retinal and choroidal yellowish infiltrates 100–300 μm in diameter. Representative fluorescein angiography of the right eye (at 40 seconds) shows prominent optic disc capillary dilation with hyperfluorescence and blockage at the site of the retinitis in the inferotemporal macula (C); the left eye (at 55 seconds) shows prominent optic disc capillary dilation as well as hypofluorescence at the site of the retinitis inferiorly (D).
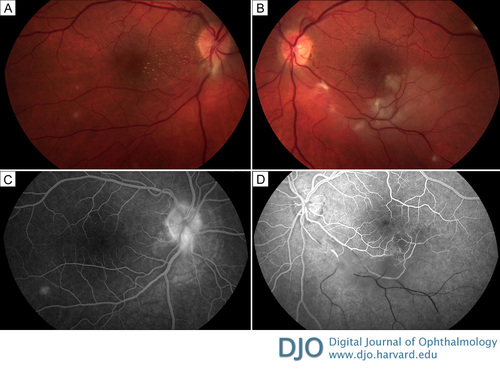
Figure 2
Fundus photographs 1 month after presentation shows stellate macular exudates (macular star) in the right eye (A) as well as a branch retinal artery and vein occlusion in the left eye (B). Representative fluorescein angiography of the right eye (at 1 minute 38 seconds) shows that the inferotemporal site of blockage has cleared (C); in the left eye (at 39 seconds) there is no perfusion distal to the branch retinal artery and vein occlusion with surrounding hypofluorescence from retinal edema present (D).
Fundus photographs 1 month after presentation shows stellate macular exudates (macular star) in the right eye (A) as well as a branch retinal artery and vein occlusion in the left eye (B). Representative fluorescein angiography of the right eye (at 1 minute 38 seconds) shows that the inferotemporal site of blockage has cleared (C); in the left eye (at 39 seconds) there is no perfusion distal to the branch retinal artery and vein occlusion with surrounding hypofluorescence from retinal edema present (D).
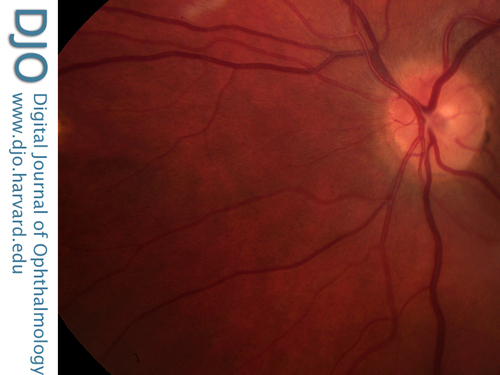
Figure 3
Fundus photograph showing the areas of retinitis becoming chorioretinal scars.
Fundus photograph showing the areas of retinitis becoming chorioretinal scars.
For neuroretinitis the main differential includes Lyme disease, malignant hypertension, syphilis, and idiopathic stellate neuroretinitis.
For the isolated retinal or choroidal infiltrates, the differential includes the white dot syndromes, particularly multiple evanescent white dot syndrome(3) and toxoplasmosis, which, unlike Bartonella infections, classically have infiltrate adjacent to chorioretinal scarring and are not multifocal.(4)
Our patient’s differential diagnosis included white dot syndromes such as multifocal choroiditis, sarcoidosis, syphilis, lyme, and infectious and idiopathic neuroretinitis.
After regional lymphadenopathy, ocular Bartonella infection is the most common manifestation of the disease. Both the presenting and final visual acuity can vary greatly, from 20/20 to counting fingers.(8) The most common ocular presentation is unilateral Parinaud oculoglandular syndrome, consisting of preauricular lymphadenopathy and follicular conjunctivitis, which was reported in 48 of 1200 patients (4%).(1) The second most common ocular finding is chorioretinal infiltrates, reported in 16 of 37 (43%) and 29 of 35 cases (83%) of ocular Bartonella infection.(10,8) Neuroretinitis (optic disc edema accompanied by a stellate pattern of exudative maculopathy) sometimes with peripapillary or equatorial dot-blot hemorrhages, is a classic finding.(8) Although neuroretinitis only occurs in 1%-2% of cases of systemic Bartonella infection,(2,9) a frequently cited study reported neuroretinitis caused by Bartonella in 9 of 14 cases (64%).(11)
Solley et al reported unilateral optic disc edema in 16 of 35 (46%) of ocular Bartonella cases.(8) Chi et al reported bilateral disc edema in only 9 of 53 patients (17%).(12) In their unilateral cases, an afferent pupillary defect was common, occurring in 40 of 44 cases (91%).(12) Optic disc edema progressed to neuroretinitis in 28 of 62 cases (45%).(12) Neuoretinitis is thought to be segmental inflammation of superficial optic nerve head arterioles leading to exudative disc edema, which spreads into the outer plexiform layer. Purvin et al reported unilateral neuroretinitis in 65 of 69 cases (94%).(13) The exudates appear 1-3 weeks after the disc edema and take 2-3 months to resolve.(14)
Omerod noted up to 27 central retinal or choroidal white dots (bilateral in an estimated 75% of cases), usually clearing completely but sometimes leaving pigmented scars: retinal disease was associated with vitritis in 50% and nongranulomatous anterior uveitis in <15% of their cases.(15) Retinal biopsy can confirm that the lesions contain Bartonella colonies.(16) In a relatively large study (n = 35), Solley et al reviewed photographs to determine the depth of infiltrates: 30% occurred in the superficial retina; 49%, in deep retina; 14% were full-thickness; and 7% were in the choroid.(8)
Branch retinal artery occlusion has been reported as frequently as in 11% of 35 cases of ocular Bartonella.(8) There is a case report of a branch retinal venous occlusion(8) and combined central retinal artery and vein occlusion.(17) Less common ocular complications include optic nerve head vasoproliferative, granulomatous angiomas that are composed of superficial abnormal vascular networks in 5% of cases.(10,18). Peripapillary serous retinal detachments, thought to be caused by spreading of fluid from the disc edema, occurred in 20% of 35 cases.(8) The subretinal fluid usually resolves after 2 months.(19) Cases of macular holes and a choroidal detachment have also been reported.(20,21)
Bartonella henselae is difficult to culture. It can be identified with the Warthin-Starry stain, but it is uncommon and usually unnecessary to culture a lymph node.(1,20) Serum indirect fluorescent antibody (IFA) with a titer ?1:64 is 88% sensitive and 94% specific,(20) except in immunocompromised patients, where the sensitivity is <70%.(2) Seroconversion requires 2-3 weeks, and a fourfold rise in titers strongly suggests the diagnosis,(21) although this only occurs in 66% of cases.(22) Although enzyme-linked immunosorbent assay (ELISA) is available to distinguish between IgG and IgM and was initially reported to be 95% sensitive and 100% specific,(21) others report that ELISA is not accurate because of high false negatives.(2,3) The combination of a papule, regional adenopathy, and a positive IFA is 95% sensitive. (22) Tissue polymerase chain reaction can be used as a confirmatory test, because it was reported to be 100% specific and 76% sensitive.(23)
Ancillary testing beyond antibody testing may offer evidence in support of positive diagnosis. Optical coherence tomography can be used to demonstrate optic disc edema and peripapillary subretinal fluid.(19) The chorioretinal infiltrates are hypofluorescent early and hyperfluorescent late on fluorescein angiography but hypofluorescent throughout indocyanine green angiography.(18)
Recommended antibiotics include ciprofloxacin (azithromycin in children),(9) sulfamethoxazole with trimethoprim, and rifampin.(24) A randomized control trial to determine the efficacy of any treatment for ocular Bartonella has not yet been reported. Although investigators have reported using antibiotics for 2-4 weeks with or without steroids,(2) neither appear to improve the visual outcome.(12) One study showed that the final median visual acuity in both treated and nontreated groups was 20/20.(8) The only cases consistently reported to require treatment are immunocompromised patients, who are at higher risk for symptomatic bacteremia. Immunocompromised patients should be treated for at least 1-4 months.(7,25,26)
Final visual acuity depends on the extent of posterior segment involvement. Chi et al reported that of 53 cases with disc edema and a mean presenting visual acuity of 20/160, 36 (68%) had a final visual acuity of 20/40 or better, and 3 (6%) had final visual acuity of 20/200 or worse.(12) While the progression from disc edema to neuroretinitis(12) or a serous retinal detachment(14) does not appear to affect final visual acuity, the presence of a vascular occlusion involving the disc or macula limits the visual outcome.(14) Purvin et al reported that disc edema and macular edema led to a central visual field deficit in 88% of tested cases,(13) but these deficits may not persist after disease resolution.(27) Reed et al reported a case series where all 7 patients finished with final visual acuity of 20/20 to 20/30: subtle residual deficits were detected 2 years later in all 3 of those patients who were tested, including decreased contrast sensitivity, a 30% reduction in the visual evoked potential amplitude, and decreased color vision.(27)
The present case demonstrates the different stages of ocular manifestation of a patient with Bartonella. Unlike previously reported cases, where artery occlusion was noted simultaneous to the retinal infiltrates,(4,15,26) our case showed an unusually progressive course. In addition, initial Bartonella serology for both IgG and IgM were negative, but repeat convalescent titers were positive for both. In patients for whom there is a high clinical suspicion of cat-scratch disease, a convalescent titer should be obtained 2-3 weeks following a negative initial result.
2. Cunningham ET, Koehler JE. Ocular bartonellosis. Am J Ophthalmol 2000;130:340-9.
3. Roe RH, Michael Jumper J, Fu AD, Johnson RN, Richard McDonald H, Cunningham ET. Ocular bartonella infections. Int Ophthalmol Clin 2008;48:93-105.
4. Cohen SM, Davis JL, Gass DM. Branch retinal arterial occlusions in multifocal retinitis with optic nerve edema. Arch Ophthalmol 1995;113:1271-6.
5. Jackson LA, Perkins BA, Wenger JD. Cat scratch disease in the United States: an analysis of three national databases. Am J Public Health 1993;83:1707-11.
6. Chomel BB, Kasten RW, Floyd-Hawkins K, et al. Experimental transmission of Bartonella henselae by the cat flea. J Clin Microbiol 1996;34:1952-6.
7. Koehler JE, Tappero JW. Bacillary angiomatosis and bacillary peliosis in patients infected with human immunodeficiency virus. Clin Infect Dis 1993;17:612-24.
8. Solley WA, Martin DF, Newman NJ, et al. Cat scratch disease: posterior segment manifestations. Ophthalmology 1999;106:1546-53.
9. Ormerod LD, Dailey JP. Ocular manifestations of cat-scratch disease. Curr Opin Ophthalmol 1999;10:209-16.
10. Curi AL, Machado D, Heringer G, et al. Cat-scratch disease: ocular manifestations and visual outcome. Int Ophthalmol 2010;30:553-8.
11. Suhler EB, Lauer AK, Rosenbaum JT. Prevalence of serologic evidence of cat scratch disease in patients with neuroretinitis. Ophthalmology 2000;107:871-6.
12. Chi SL, Stinnett S, Eggenberger E, et al. Clinical characteristics in 53 patients with cat scratch optic neuropathy. Ophthalmology 2012;119:183-7.
13. Purvin V, Sundaram S, Kawasaki A. Neuroretinitis: review of the literature and new observations. J Neuroophthalmol 2011;31:58-68.
14. Wade NK, Levi L, Jones MR, Bhisitkul R, Fine L, Cunningham ET. Optic disk edema associated with peripapillary serous retinal detachment: an early sign of systemic Bartonella henselae infection. Am J Ophthalmol 2000;130:327-34.
15. Ormerod LD, Skolnick KA, Menosky MM, Pavan PR, Pon DM. Retinal and choroidal manifestations of cat-scratch disease. Ophthalmology 1998;105:1024-31.
16. Warren K, Goldstein E, Hung VS, Koehler JE, Richardson W. Use of retinal biopsy to diagnose Bartonella (formerly Rochalimaea) henselae retinitis in an HIV-infected patient. Arch Ophthalmol 1998;116:937-40.
17. Gray AV, Michels KS, Lauer AK, Samples JR. Bartonella henselae infection associated with neuroretinitis, central retinal artery and vein occlusion, neovascular glaucoma, and severe vision loss. Am J Ophthalmol 2004;137:187-9.
18. Matsuo T, Yamaoka A, Shiraga F, et al. Clinical and angiographic characteristics of retinal manifestations in cat scratch disease. Jpn J Ophthalmol 2000;44:182-6.
19. Habot-Wilner Z, Zur D, Goldstein M, et al. Macular findings on optical coherence tomography in cat-scratch disease neuroretinitis. Eye (Lond) 2011;25:1064-8.
20. Seth A, Raina U, Thirumalai S, Batta S, Ghosh B. Full-thickness macular hole in Bartonella henselae neuroretinitis in an 11-year-old girl.Oman J Ophthalmol 2015;8:44-6.
21. Anders U, Taylor E, Doty D, Martel J, Martel J. Neuroretinitis secondary to Bartonella henselae in the emergent setting. Epub ahead of print, December 2, 2014. Am J Emerg Med.
20. Regnery RL, Olson JG, Perkins BA, Bibb W. Serological response to “Rochalimaea henselae” antigen in suspected cat-scratch disease. Lancet 1992;339:1443-5.
21. Barka NE, Hadfield T, Patnaik M, et al. EIA for detection of Rochalimaea henselae–reactive IgG, IgM, and IgA antibodies in patients with suspected cat-scratch disease. J Infect Dis 1993;167:1503-4.
22. Dalton MJ, Robinson LE, Cooper J, et al. Use of Bartonella antigens for serologic diagnosis of cat-scratch disease at a national referral center. Arch Intern Med 1995;155:1670-6.
23. Hansmann Y, DeMartino S, Piémont Y, et al. Diagnosis of cat scratch disease with detection of Bartonella henselae by PCR: a study of patients with lymph node enlargement. J Clin Microbiol 2005;43:3800-6.
24. Margileth AM. Antibiotic therapy for cat-scratch disease: clinical study of therapeutic outcome in 268 patients and a review of the literature. Pediatr Infect Dis J 1992;11:474-8.
25. Adal KA, Cockerell CJ, Petri WA. Cat scratch disease, bacillary angiomatosis, and other infections due to Rochalimaea. N Engl J Med 1994;330:1509-15.
26. Jones MR, Cunningham ET. Bartonella henselae–associated acute multifocal retinitis in a patient with acquired immunodeficiency syndrome. Retina 1997;17:457-9.
27. Reed J, Scales D, Wong M, Lattuada C, Dolan M, Schwab I. Bartonella henselae neuroretinitis in cat scratch disease. Diagnosis, management, and sequelae. Ophthalmology 1998;105:459-66.