A 48-year-old woman with redness, photophobia, and eye discomfort
Digital Journal of Ophthalmology 2013
Volume 19, Number 2
May 1, 2013
DOI: 10.5693/djo.03.2013.01.003
Volume 19, Number 2
May 1, 2013
DOI: 10.5693/djo.03.2013.01.003
Download PDF
Slit-lamp examination of the left eye was notable for 2+ ciliary injection and for 2+ anterior chamber cell and flare accompanied by scattered mutton fat and stellate keratic precipitates in Arlt’s triangle. No hypopyon was observed. She was noted to have an intumescent cataract with a prominent vertical cleft (Figure 1). There was no anterior bowing of the iris, and the angle was open to the scleral spur without synechiae on gonioscopy.
The anterior segment examination of her right eye was unremarkable. Dilated fundus examination revealed a sharp, pink disc with a 0.4 cup-to-disc ratio and a healthy fundus. There was no posterior view in the left eye due to the density of her cataract.
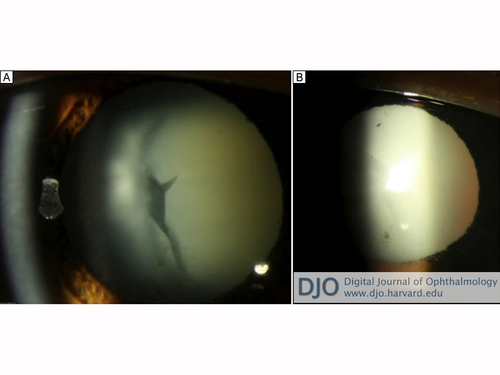
Figure 1
A, Intumescent cataract with prominent vertical cleft. B, Retro-illumination of cornea demonstrating stellate and mutton fat keratic precipitates.
A, Intumescent cataract with prominent vertical cleft. B, Retro-illumination of cornea demonstrating stellate and mutton fat keratic precipitates.
Consideration of infectious endophthalmitis from an indolent organism such as Propionibacterium acnes, Staphylococcus epidermidis, or Candida parapsilosis was also warranted because, while such infections do not usually produce granulomatous inflammation, the consequences of missing the diagnosis can be severe. Such infections can initially appear to respond to steroids, so the patient’s clinical improvement did not entirely rule them out. Again, however, the patient’s clinical presentation and B-scan findings were not typical, and the rates of endophthalmitis after pars plana vitrectomy are quite low with an incidence of approximately 0.02% in a retrospective study by the Pan American Collaborative Retina Study Group.(1)
Given the presence of an intumescent cataract, lens-related etiologies were also carefully considered. Phacomorphic glaucoma was ruled out by confirming that the anterior chamber angle was open on gonioscopy. Phacolytic glaucoma, which involves the leakage of soluble lens proteins through an intact capsular bag in the setting of a mature cataract and usually results in nongranulomatous anterior segment inflammation, was thought less likely than phacoantigenic endophthalmitis, a classically granulomatous autoimmune response to lens proteins. The rapid postoperative development of cataract in our patient might have been due to occult lens capsule trauma during the pars plana vitrectomy.
Phacoantigenic endophthalmitis is a rare granulomatous uveitis caused by altered immune tolerance to lens proteins following capsular disruption. The traditional term, “phacoanaphylactic endophthalmitis,” is misleading because inflammation results from immune complex formation rather than IgE crosslinking and histamine release. When first described by Straub in 1919, basic immunologic mechanisms were not yet understood, and “anaphylaxis” was used to describe all sudden-onset inflammation.(2,3)
Until the 1980s, it was erroneously believed that the immune system attacked antigens previously sequestered by the lens capsule. Lens-related antigens have since been identified in organs throughout the body and lens proteins are known to be present in low concentrations in the aqueous of normal individuals.(4) There are many cases in which the lens capsule is disrupted without inciting inflammation. Disruption is therefore necessary but not alone sufficient to cause an immunologic response. The exact stimulus for alloimmunization remains unclear, but the process of immune complex formation and inflammatory cell recruitment has been well elucidated.(5)
Ultrasound findings consistent with a fulminant infectious endophthalmitis include abundant vitreous debris, often in loculated pockets, inflammatory membranes, and occasionally retinal or choroidal detachment. While B-scans in early endophthalmitis may not demonstrate classical findings, this paucity would be unusual in a patient presenting within 10 days of symptoms.
Phacoantigenic endophthalmitis most often follows surgical or traumatic penetration of the lens capsule but also has been described after spontaneous capsular rupture resulting from advanced, swollen cataracts. Inflammation often develops within days to weeks of the inciting event, but latency periods of up to 59 years have been reported.(4) Peak incidence is in the fifth to seventh decades despite higher rates of ocular trauma in younger individuals.(2,4)
Though rare, phacoantigenic endophthalmitis seems to be an under-recognized condition. In a series of 144 cases of histopathologically confirmed phacoantigenic endophthalmitis, Thatch et al found that the pre-enucleation diagnosis was correct in only 5% of cases.(2) Clinically, phacoantigenic endophthalmitis can be difficult to distinguish from other forms of postoperative uveitis. Patients often present with photophobia and ciliary reaction. Findings can range from mild anterior uveitis to fulminant endophthalmitis with hypopyon. Mutton fat keratic precipitates and peripheral anterior synechiae are characteristic but not universal. Most manifestations of phacoantigenic endophthalmitis are anterior, but late sequellae may include mononuclear choroiditis, optic atrophy, retinal perivasculitis, or retinal detachment.(4)
If clinical suspicion is high, early evaluation and treatment for infectious endophthalmitis is critical. Several recent studies report the utility of anterior chamber paracentesis for cytologic evaluation and/or western blot quantification of lens protein concentration, but these procedures are not standard clinical practice.(6,7) Histopathology is not practical or necessary in many cases, but when performed, it typically reveals a zonal granulomatous pattern with concentric rings of polymorphonuclear leukocytes, epithelioid and giant cells, and mononuclear cells.
In cases of granulomatous anterior uveitis associated with lens capsule disruption, patients are initially treated with steroids. When only small lens fragments are retained, as after phacoemulsification, inflammation may subside with medical management alone. When phacoantigenic endophthalmitis follows lens trauma, as with our patient, surgery is required for definitive treatment.
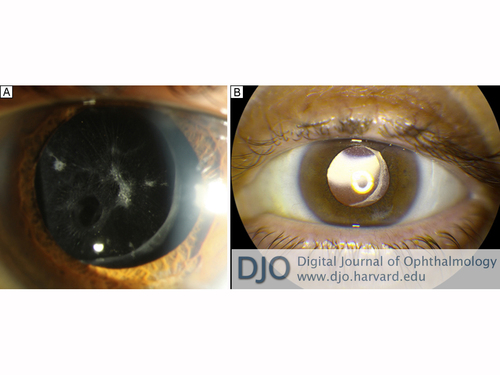
Figure 2
A, Oval hole in the inferonasal posterior capsule from unrecognized intraoperative vitrector trauma. B, Result after placement of posterior chamber intraocular lens.
A, Oval hole in the inferonasal posterior capsule from unrecognized intraoperative vitrector trauma. B, Result after placement of posterior chamber intraocular lens.
2. Thach AB, Marak GE Jr, McLean IW, Green WR. Phacoanaphylactic endophthalmitis: a clinicopathologic review. Int Ophthalmol 1991;15:271-9.
3. Perlman EM, Albert DM. Clinically unsuspected phacoanaphylaxis after ocular trauma. Arch Ophthalmol 1977;95:244-6.
4. Marak GE Jr. Phacoanaphylactic endophthalmitis. Surv Ophthalmol 1992;36:325-39.
5. Till GO, Lee S, Mulligan MS, et al. Adhesion molecules in experimental phacoanaphylactic endophthalmitis. Invest Ophthalmol Vis Sci 1992;33:3417-23.
6. Tanito M, Kaidzu S, Katsube T, Nonoyama S, Takai Y, Ohira A. Diagnostic Western blot for lens-specific proteins in aqueous fluid after traumatic lens-induced uveitis. Jpn J Ophthalmol 2009;53:436- 9.
7. Hochman M, Sugino IK, Lesko C, Friedman AH, Zarbin MA. Diagnosis of phacoanaphylactic endophthalmitis by fine needle aspiration biopsy. Ophthalmic Surg Lasers 1999;30:152-4.