A 7-year-old boy with a right anterior orbital mass
Digital Journal of Ophthalmology 2010
Volume 16, Number 3
August 30, 2010
DOI: 10.5693/djo.03.2010.02.001
Volume 16, Number 3
August 30, 2010
DOI: 10.5693/djo.03.2010.02.001
Download PDF
• Dermoid and simple epithelial cysts are the most common orbital lesions in the pediatric population. In a series of 250 cases by Shields et al., dermoid cysts accounted for 46% of childhood orbital lesions and 89% of all cystic lesions.(1) These figures may be even higher in the practice of a general ophthalmologist. The majority of these cystic lesions are superficial and confined to the anterior orbit. Superficial dermoid cysts are diagnosed clinically by their typical presentation: a non-tender, mobile mass at the outer orbital rim in a young child.(2) They are best removed intact by an anterior approach through a superior eyelid crease incision.(3) Deep dermoid cysts are detected at a later age, and they are in surgically challenging locations often requiring a lateral orbitotomy with a higher risk of injury to orbital and ocular tissues.
• Orbital mucoceles are secondary cysts. They arise from chronically inflamed paranasal sinuses, and they usually develop in adulthood, except in patients with cystic fibrosis where a presentation in childhood is possible.
• Parasitic cysts such as cysticercosis and hydatid cysts occur usually in endemic areas such as Mexico, Central and South America, and India.
• Inflammatory lesions accounted for 16% in the series by Shields et al.; they are usually biopsied to exclude the diagnosis of rhabdomyosarcoma.(1)
• Although rhabdomyosarcoma is not a common orbital space-occupying lesion in children, one should be familiar with its clinical features: sudden onset of painless proptosis with impairment of ocular motility and non-axial displacement of the globe. Immediate biopsy is indicated. In the above-mentioned case series, there were 14 primary malignant tumors (6%), 10 of which were rhabdomyosarcoma.(1)
Histological examination revealed a fibrous pseudo-capsule that surrounded a retracted and collapsed cyst (Figure 1A). The fibrous pseudo-capsule showed collagenous tissue with few inflammatory cells (lymphocytes and segmented eosinophils) and a layer of plump histiocytes facing the cyst (Figure 1B, upper half), which was consistent with a host reaction. The cyst consisted of an outer layer of homogenous eosinophilic material (tegument) supported by a row of viable cells (tegumental cells) with clear cytoplasm and small round nuclei (Figure 1B, lower half). The myxoid material noted on gross examination was actually formed tissue, i.e. very loose edematous stroma that along with free fluid comprised the contents of the cyst; this was suggestive of a larva. In another part of the glass slide, the protoscolex (diagnostic element of the larva that fell out of the cyst during dissection) was identified (Figure 2). It measured 0.3 cm and presented spiral stacks of thick invaginated tegument typical of a cestode larva. The diagnosis was narrowed to larva of Taenia solium (T. solium), a cysticerus because this was the only scolex found in the cyst (4); multiple scolices would be diagnostic of a coenurus, the larval stage of T. multiceps (tapeworm of the dog).
The life cycle of the tapeworm T. solium consists of two stages:
1. The larval stage is initiated when a pig (intermediate host) ingests mature T. solium eggs from pastures and barnyards contaminated with human feces. The eggs hatch, and the ensuing larvae migrate through the bowel wall into the bloodstream. They establish encysted larvae (cysticerci) in various organs, especially muscle tissues.
2. The mature stage commences when a human (definitive host) ingests the encysted larvae in the form of poorly cooked pork. The larvae attach to the mucosa of the small bowel and develop into mature tapeworms. The life cycle of T. solium is completed when a mature worm releases gravid proglottids (worm segments) full of eggs into the stools, which contaminate the environment in areas of the world with poor sanitation. The mature stage, known as intestinal taeniasis, produces mild or no clinical symptoms; however, the infection tends to persist for many years. Infective eggs may also be recovered from fingernail dirt, skin, and clothes of carriers.(5)
Unfortunately, humans are susceptible to becoming intermediate, albeit dead-end, hosts in the T. solium life cycle. This condition, known as cysticercosis, is not acquired from eating pork. It is very serious when the larvae encyst in the brain (neurocysticercosis) or the orbit.(6) Human ingestion of taenia eggs may be linked to contaminated food or water, or via person-to person transmission of infective eggs through the oral-fecal route. In the United States, most cases of cysticercosis are diagnosed among immigrants and seasonal workers from rural areas of Latin America, Asia, and Africa with endemic cysticercosis. However, cases of locally acquired cysticercosis have been documented in several states.(5,6) Interestingly, orbital cysticercosis has a special predilection for children and young adults and should be included in the differential diagnosis of dermoid cysts. Knowledge of the natural course of the cysticercosis is important while conducting the diagnostic work-up of orbital cysts. Medical and clinical history should elicit whether the patient emigrated from an endemic area, visited an endemic area, or was in contact with a person with taeniasis. The presence of subcutaneous nodules or neurological symptoms should also be noted. Laboratory work-up would include stools testing for ova and parasites by direct and concentration methods, search for peripheral eosinophilia, and serum screening for cysticercosis antibody preferably by immunoblot assay. A positive result for any of these tests is helpful but negative results do no rule out the disease; it is well known that serology may be negative in patient with one or few cysts.
The diagnosis of cysticercosis can be based solely on orbital imaging if performed:
• A live cyst produces a mass effect with minimal inflammatory reaction, and the eccentric protoscolex can be identified.
• A dying larva shows a retracted cyst with prominent local inflammatory reaction that later leads to fibrosis and calcification.
The live cyst is better identified on MRI, while fibrosis and calcifications are better seen on CT scan.
Due to recent encouraging results of medical therapy for orbital cysticercosis, there is a need to reasonably exclude cysticercosis prior to the attempt of any complicated surgical procedure. Surgery is advocated for easily accessible cysts, such as those in the superficial anterior orbit, subcutaneous eyelid, or conjunctiva. Of note, one should avoid opening the cyst during surgery, and if aspiration is deemed necessary, the surgical area should be irrigated with hypertonic saline. Antihelminthic therapy should be considered after surgery.
Impairment of ocular motility denotes myocysticercosis, impairment of extraocular muscle involvement. This is the commonest form of orbital cysticercosis, and a tissue diagnosis is not essential for initiating the preferred therapeutic modality, that is, medical therapy, consisting of a combination of albendazole—an antihelminthic that kills the parasite by blocking its uptake of glucose—and corticosteroids, which are essential for the suppression of the acute inflammatory reaction that follows the death of the parasite and the release of the cyst contents. Adequate control of the inflammatory reaction is crucial for the prevention of the fibrosis, which can reduce ocular motility. Albendazole is a well-tolerated drug and is effective in up to 95% of myocysticercosis cases.(7) Injection of the cyst with absolute alcohol may be considered as an option in the event of the failure or unsuitability of the antihelminthic drug.
Follow-up
Further questioning of the family revealed that the mother was originally from Mexico, and the boy had visited Mexico on several holiday trips. The patient and his family underwent stool examination for ova and parasites and serologic screening for cysticercosis antibody by immunoblot assay; all screening tests were negative. Fortunately, a repeat ophthalmological examination and an MRI of the brain of the child were negative; the MRI was also compared to an earlier MRI of the brain performed at the time of the seizure episodes at the age of 6 months. Following a consultation with a parasitologist, the child was offered a two-week course of albendazole.
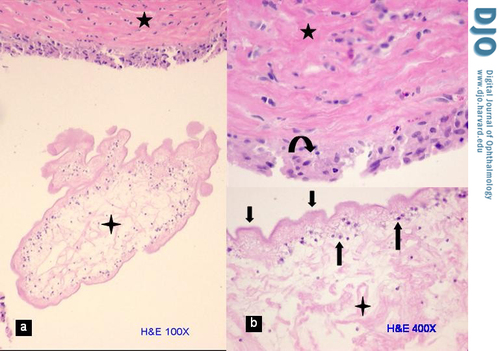
Figure 1
Cyst wall. A, Photomicrograph of a portion of the collapsed and retracted cysticercus (4-point star) within the fibrous pseudo-capsule of the host (5-point star) with minimal inflammatory reaction (hematoxylin-eosin stain, original magnification 100×). B, Higher magnification of same showing the three layers of the cysticercus— loose stroma (4 point star), tegumental cells (up arrows), and wavy tegument (down arrows)—and the fibrous pseudo-capsule (5 point star) with a layer of histiocytes (curved arrow) facing the cysticercus (original magnification 400×).
Cyst wall. A, Photomicrograph of a portion of the collapsed and retracted cysticercus (4-point star) within the fibrous pseudo-capsule of the host (5-point star) with minimal inflammatory reaction (hematoxylin-eosin stain, original magnification 100×). B, Higher magnification of same showing the three layers of the cysticercus— loose stroma (4 point star), tegumental cells (up arrows), and wavy tegument (down arrows)—and the fibrous pseudo-capsule (5 point star) with a layer of histiocytes (curved arrow) facing the cysticercus (original magnification 400×).
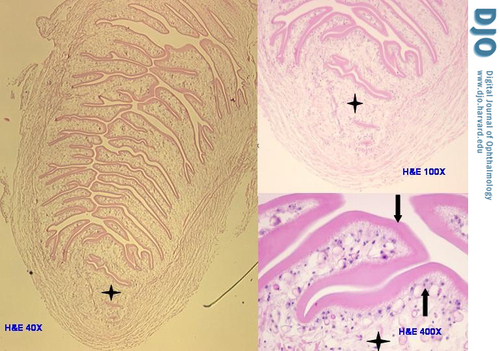
Figure 2
Photomicrograph depicts the only protoscolex of the cysticercus (left panel), which fell out of the loose stroma of the cyst during dissection (hematoxylin-eosin stain original magnification 40×). At higher magnifications (right panel) the protoscolex shows the same three layers of the rest of the cysticercus—loose stroma (4-point star), tegumental cells (up arrow), and wavy tegument (down arrow)—however, the much thicker tegument is invaginated and stacked within the protoscolex (upper right original magnification 100×, and lower right original magnification 400×)
Photomicrograph depicts the only protoscolex of the cysticercus (left panel), which fell out of the loose stroma of the cyst during dissection (hematoxylin-eosin stain original magnification 40×). At higher magnifications (right panel) the protoscolex shows the same three layers of the rest of the cysticercus—loose stroma (4-point star), tegumental cells (up arrow), and wavy tegument (down arrow)—however, the much thicker tegument is invaginated and stacked within the protoscolex (upper right original magnification 100×, and lower right original magnification 400×)
2. Ahuja R, Azar NF. Orbital Dermoids in Children. Semin Ophthalmol 2006;21:207-11.
3. Shields JA, Shields CL. Orbital cysts of childhood—classification, clinical features, and management. Surv Ophthalmol 2004;49:281-99.
4. Cestodes, Taenia solium et Taenia saginata. Deschiens, V. - Atlas de parasitologie, Paris. Page Available via DIALOG http://www.bium.univ-paris5.fr/histmed/medica/page?351637&p=36. Accessed August 15th 2009.
5. Kraft R. Cysticercosis: an emerging parasitic disease. Am Fam Physician 2007;76:91-6.
6. Locally Acquired Neurocysticercosis: North Carolina, Massachusetts, and South Carolina, 1989-1991. MMWR 1992;41:1-4.
7. Pushker N, Bajaj MS, Betharia SM. Orbital and adnexal cysticercosis. Clin Experiment Ophthalmol 2002;30:322–33.