A 26-year-old man with renal failure and vision loss
Digital Journal of Ophthalmology 2008
Volume 14, Number 13
July 12, 2008
Volume 14, Number 13
July 12, 2008
The vital signs upon presentation are summarized in Table 1. The patient’s physical exam showed bilateral costovertebral angle tenderness, and his laboratory tests are summarized in Table 2. A computed tomography scan was negative for nephrolithiasis, an electrocardiogram and an echocardiogram showed left ventricular hypertrophy, and a renal ultrasound showed small and echogenic kidneys bilaterally.
The patient was diagnosed with nephrotic syndrome and end-stage renal disease (ESRD). An extensive workup was carried out to define the etiology of the renal failure (Table 3). A kidney biopsy was not warranted given the ESRD and the potential complications associated with the procedure. His anemia was though to be anemia of chronic disease secondary to renal failure and his dyslipidemia is consistent with nephrotic syndrome.
He was started on hemodialysis three times a week, plus Darbepoetin Alfa and Simvastatin for anemia and dyslipidemia, respectively. The patient was compliant with the hemodialysis schedule and the prescribed medications.
One year later, the patient presented to the emergency room complaining of severe headache and vomiting followed by sudden vision loss bilaterally. His vital signs are summarized in Table 4. A computed tomography scan of the head showed no evidence of bleeding, midline shift, or mass. A lumbar puncture was performed and acute meningitis was ruled out.

Table 1
Vital signs at initial presentation
Vital signs at initial presentation
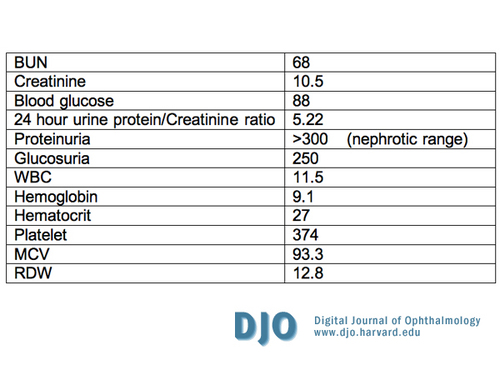
Table 2
Laboratory Tests
Laboratory Tests
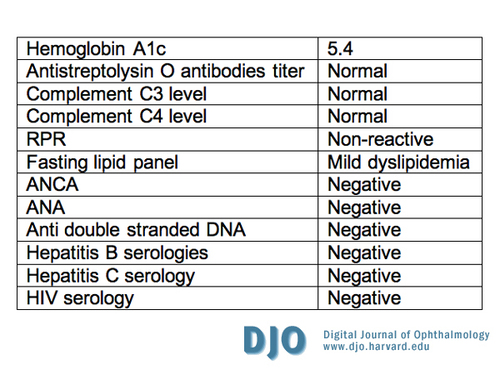
Table 3
Laboratory work-up for cause of renal failure
Laboratory work-up for cause of renal failure

Table 4
Vital signs at second presentation
Vital signs at second presentation
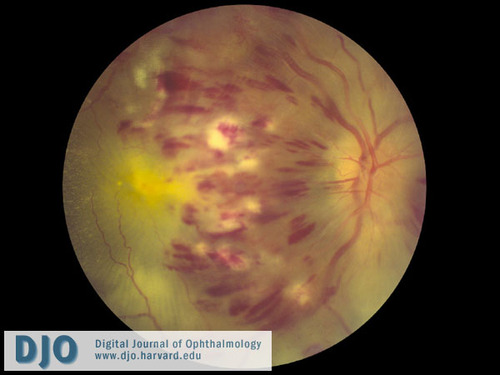
Figure 1
Color fundus photograph of the right eye shows extensive intraretinal hemorrhages in all 4 quadrants, dilated and tortuous retinal veins, cotton wool spots, and disc edema.
Color fundus photograph of the right eye shows extensive intraretinal hemorrhages in all 4 quadrants, dilated and tortuous retinal veins, cotton wool spots, and disc edema.
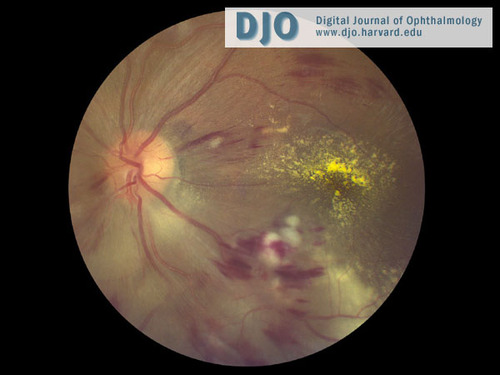
Figure 2
Color fundus photograph of the left eye shows severe inferior disc swelling, intraretinal hemorrhages, and cotton-wool spots.
Color fundus photograph of the left eye shows severe inferior disc swelling, intraretinal hemorrhages, and cotton-wool spots.
FA of the left eye (65 seconds post fluorescein injection) revealed blockage of choroidal fluorescence by intraretinal heme, leakage correlating with areas of retinal edema, and areas of non-perfusion inferiorly (Figure 4).
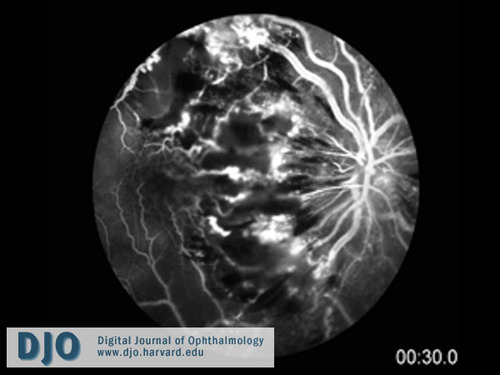
Figure 3
Fluorescein angiogram of the right eye (at 30 seconds post fluorescein injection) showing extensive blockage of choroidal fluorescence by intra-retinal hemorrhage and edema, leakage correlating with areas of retinal edema, venous engorgement and tortuosity, and extensive non-perfusion involving the macula and mid-periphery.
Fluorescein angiogram of the right eye (at 30 seconds post fluorescein injection) showing extensive blockage of choroidal fluorescence by intra-retinal hemorrhage and edema, leakage correlating with areas of retinal edema, venous engorgement and tortuosity, and extensive non-perfusion involving the macula and mid-periphery.
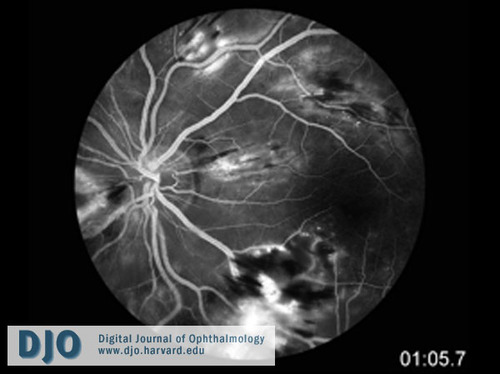
Figure 4
Fluorescein angiogram of the left eye (at 65 seconds post fluorescein injection) showing blockage of choroidal fluorescence by intra-retinal hemorrhage, leakage correlating with areas of retinal edema, and areas of non-perfusion inferiorly.
Fluorescein angiogram of the left eye (at 65 seconds post fluorescein injection) showing blockage of choroidal fluorescence by intra-retinal hemorrhage, leakage correlating with areas of retinal edema, and areas of non-perfusion inferiorly.
Complete hematologic and coagulation workup came back negative (Table 5), but the homocysteine and CRP were both elevated. The patient was followed closely by ophthalmology for the first 6 months, including gonioscopy and undilated examination of the iris, to monitor for signs of neovascularization of the iris and neovascular glaucoma.
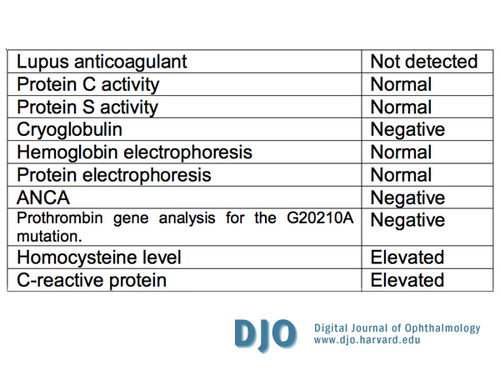
Table 5
Coagulopathy workup
Coagulopathy workup
2. Ocular ischemic syndrome (carotid occlusion disease):
In ocular ischemic syndrome, the veins are usually dilated and irregular (but not tortuous). Although revascularization of the disc is present in one third of the cases, disc edema and hemorrhages are not characteristic. Retinal hemorrhages tend to be in the mid periphery. Patients may have a history of amaurosis fugax, transient ischemic attacks, or orbital pain. The intra-ocular pressure is often low as is the central retinal artery perfusion pressure.
3. Diabetic retinopathy:
For diabetic retinopathy, hemorrhages and microaneurysms are usually concentrated in the posterior pole, exudates are more prominent, and the condition is typically bilateral. Intravenous flourescein angiography may be required to distinguish this condition from central retinal vein occlusion.
4. Papilledema:
This is characterized by bilateral disc swelling with flame-shaped hemorrhages surrounding the disc but not extending to the peripheral retina. It results from increased intracranial pressure.
5. Radiation retinopathy:
A history of irradiation is critical for this diagnosis. Disc swelling with radiation papillopathy, and retinal neovascularization may be present. Generally, cotton-wool spots are a more prominent feature than hemorrhages.
6. Acute hypertensive retinopathy:
The blood pressure at presentation is critical for the diagnosis. It is characterized by focal or generalized vasoconstriction and breakdown of the blood-retinal barrier, with subsequent hemorrhages and exudates. Findings include:
• Retinopathy: This is characterized by arteriovenous nicking, copper or silver wire arterial changes, hemorrhages, exudates, and cotton wool spots.
• Choroidopathy: This is characterized by fibrinoid necrosis of choroidal arterioles. There may be Elschnig’s spots (zones of nonperfusion of the choriocapillaris with pale white or red patches of the retinal pigment epithelium) and exudative retinal detachment. This is often due to an acute hypertensive episode (preeclampsia, eclampsia, or pheochromocytoma). FA shows early hypoperfusion and late staining.
• Optic neuropathy: This is characterized by florid disc edema with macular exudates.
The pathology is characterized by thickening of arteriolar walls, which leads to nicking of venules and endothelial hyperplasia. Complications of hypertensive retinopathy include retinal vein occlusion, retinal macroaneurysm, nonarteritic anterior ischemic optic neuropathy, ocular motor nerve palsies, and worsening of diabetic retinopathy.
Complete hematological and coagulation evaluations were performed (summarized in table 5). The homocysteine level was found to be elevated, and this is associated with a hypercoagulable state in nephrotic syndrome. The C-reactive protein was elevated as well. A rheumatology consult was not entertained given the clinical picture, pertinent negative review of systems, and the negative laboratory workup. The patient was followed closely by ophthalmology for six months, including gonioscopy and undilated examination of the iris. Serial exams showed no signs of neovascularization of the iris or neovascular glaucoma. The patient, however, was lost to follow up due to a prolonged admission to an outside hospital for a dialysis catheter infection. The patient was called and encouraged to follow up once his acute line infection issues resolve.
Central retinal vein occlusion typically occurs in people over 50 years of age. Major risk factors are hypertension, diabetes mellitus, and atherosclerosis. Other risk factors are glaucoma, syphilis, sarcoidosis, vasculitis, increased intraorbital or intraocular pressure, hyphema, hyperviscosity syndromes (multiple myeloma, Waldenstromʼs macroglobulinemia, and leukemia), high homocysteine levels, sickle cell, and HIV. Younger patients, especially those less than 45 years of age, who present with central retinal vein occlusion should have further hematologic and coagulation workup to rule-out an underlying thrombotic disorder.(1)
CRVO has two types:
• The Nonischemic type (incidence of 70%) is defined as <10 disk diameters (DD) of capillary nonperfusion. It is characterized by vision that is better than 20/200. Among this group, 16% progress to nonperfused CRVO and 50% resolve completely without treatment.
• The Ischemic type (incidence of 30%) is defined as more than 10 DD of nonperfusion. Patients are usually older and have worse vision. Iris neovascularization is seen in 60%. Up to 33% develop neovascular glaucoma. Ten percent are combined with branch retinal arterial occlusion (usually the cilioretinal artery is occluded due to low perfusion pressure of the choroidal system).(2)
The central vein occlusion study (CVOS) data did not support the recommendation for prophylactic panretinal photocoagulation (PRP). The CVOS found that early PRP decreased the rate of iris neovascularization (INV); however, the reduction was not statistically significant. Moreover, the study showed that early PRP reduced, but did not eliminate, the possibility of anterior-segment neovascularization. The CVOS recommended close follow-up of eyes with CRVO during the first 6 months (including gonioscopy and undilated slit-lamp examination of the iris) and prompt PRP of eyes in which iris neovascularization (INV) or angle neovascularization (ANV) develops.(3)
Risk factors for developing iris neovascularization in patients with CRVO are the amount of nonperfused retina, extent of retinal hemorrhages, male sex, and central vein occlusion of less than one month duration.(3) The baseline visual acuity of patients with CRVO is a strong predictor for the development of INV/ANV, as is the amount of nonperfusion seen by fluorescein angiogram.(4)
Although PRP was better than selective PRP or photodynamic therapy at preventing INV and anterior segment neovasculariation progression, selective PRP or PDT can also be safely used to treat anterior segment neovascularization secondary to ischemic CRVO.(5)
Every eye with CRVO is at risk for developing neovascular glaucoma. Lowering intraocular pressure helps to improve retinal circulation in an eye with CRVO(6), and there is a 10 % risk for development of BRVO or CRVO in the fellow eye.(7)
This case illustrates the potential importance of regular eye exams in young patients with end-stage renal disease on hemodialysis. Young patients with nephrotic syndrome and end-stage renal disease can develop CRVO.
2. Freidman N, Kaiser P, and Trattler W. Review of ophthalmology. Philadephia: Elsevier Saunders, 2005.
3. The Central Vein Occlusion Study Group. A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. The central vein occlusion study group N report. Ophthalmology. 1995 Oct;102 (10):1434-44.
4. The Central Vein Occlusion Study Group. Natural history and clinical management of central retinal vein occlusion. Arch Ophthalmol. 1997 Apr;115(4):486-91.
5. Parodi MB, Friberg TR, Pedio M, Fiotti N, Di Stefano G, Ravalico G. Panretinal photocoagulation and photodynamic therapy for anterior segment neovascularization secondary to ischemic central retinal vein occlusion. Ophthalmic Surgery, Lasers, and Imaging. 2007 Mar-Apr;8(2):94-9.
6. Hayreh SS. Prevalent misconceptions about acute retinal vascular occlusive disorders. Prog Retin Eye Res. 2005 Jul; 24(4): 493-519.
7. Central Vein Occlusion Study Group. Baseline and early natural history report. The Central Vein Occlusion Study. Arch Ophthalmol. 1993;111:1087-95.